Standards, interoperability and regulation in health informatics
Extent
5 ECTSCourse dates
Application period
Fees
Language of instruction
Study fields
Organising unit
Mode of study
Study level
Extensive information package on regulations, standards and interoperability in health informatics and software development
An increasing variety of information systems and software are being developed for healthcare needs. Because many of them process patient data or are directly related to, for example, diagnosis, they are strictly regulated by the authorities. When developing such products, you need to know and understand the regulations and standards in force.
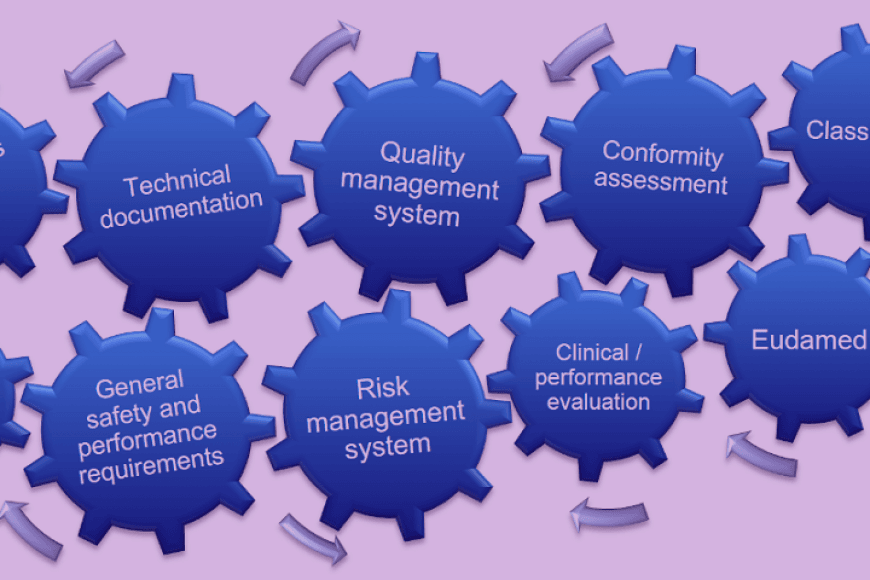
This course delves into those regulations, requirements, and standards. Additionally, system's interoperability, security and data protection are the central themes of the course. The course also introduces the quality and risk management systems required in the software production in the field and cyber security requirements.
The course covers the following topics:
- Health informatics regulations, particularly for health software production: key international directives, national regulation, self-regulation.
- Security, privacy, and data protection. Principles, regulation and guidelines for application in health informatics.
- Standards and interoperability in health informatics. Key standards: HL7, CDA, IHE, etc.
- Health information systems conformance assessment.
After completing this course, you:
- can define what data security, privacy and safety
- know the central regulations related to health information systems, privacy, and security of health data, and know how they need to be taken into account in health information system development
- know general health informatics standards and interoperability approaches and can describe how they are applied in practice
- can apply the most important standards which are widely used in Finland in health information systems in software development
- can assess whether a health information system is implemented according to regulations and interoperability principles
Teaching
The course includes an introductory live lecture, a series of video lectures and assignments.
Assignments are done in joint exercise sessions in Tampere at Hervanta campus, typically in small groups, but it is possible to do them remotely as individual exercises as well. Each particant also prepares a personal mind map of the course as a learning assignment.
The course can be completed entirely online.
The teacher of the course is Alpo Värri.
Alpo Värri has been involved in the standardization of healthcare information technology since the 1990s.
He chairs the WG2 Technology and applications working group TC251 Health Informatics of the European Committee for Standardization CEN Technical Committee. In addition, he has participated in the development of several standards in the ISO / TC215 and IEEE 11073 standardization committees.
Target group
The course is targeted to those who are working or willing to work in health software development companies or health information system management roles in health delivery organizations. The course gives essential knowledge also for health technology start-ups and anyone who wants to update his or her knowledge about standards, interoperability, and regulations in health informatics.
Basic knowledge of healthcare information systems, software production and communication protocols are an advantage in the course.