ProtMarket
ProtMarket
Faculty of Medicine and Health Technology has established protein expression and purification service that manufactures recombinant proteins for academic and industrial partners. We have capability to express proteins with E. coli, insect cells (Sf9 and Hi5) and cell free systems depending from the need.
We have good variety of purification methods and instruments available to purify and characterize the proteins.
General information
Proteins are recombinant proteins expressed in E. coli except Fibronectin witch is purified from human plasma. All proteins are purified with one-step affinity chromatography, dialyzed and analyzed with SDS-PAGE. Concentration is measured by UV/Vis spectroscopy. Thereafter proteins are lyophilized and kept -80 °C until shipment in ambient temperature.
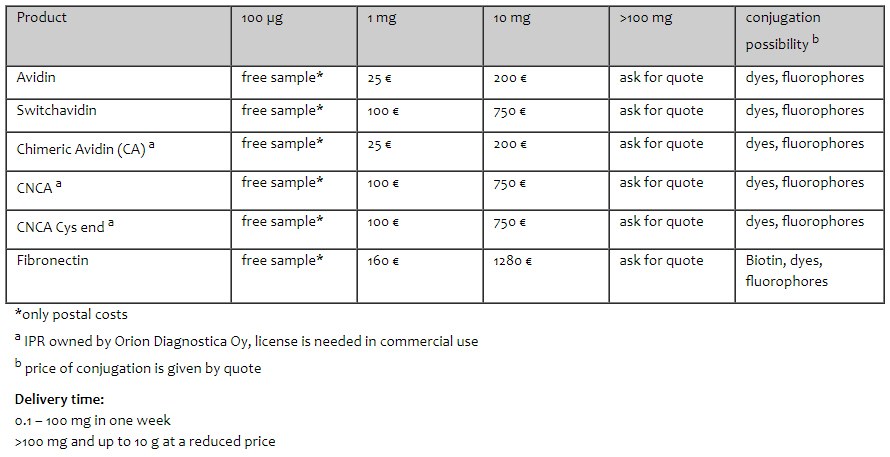
RECOMBINANT AVIDIN
SwitchAVIDIN
CHIMERIC AVIDIN
CHARGE-NEUTRALIZED CHIMERIC AVIDIN
CHARGE-NEUTRALIZED CHIMERIC AVIDIN with linkable cysteine in C-terminal
Fibronectin