Regulatory training is necessary already at the R&D stage
PhD Mikael Turunen works at Algoa Progress, a joint venture that grew out of research conducted at the University of Eastern Finland. The company develops software for modelling the progression of osteoarthritis.
“After graduating with a doctorate, I worked as an academy research fellow before joining Algoa Progress a couple of years ago,” Turunen says.
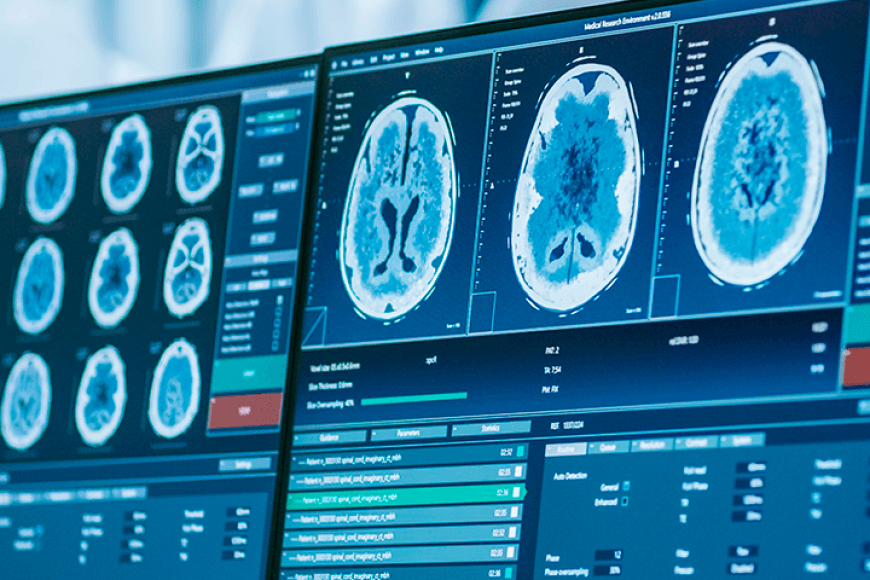
A team of researchers at Algoa Progress is developing a computational model for predicting the progression of osteoarthritis in individual patients. What is required is a knee MRI or x-ray, which shows the anatomical measurements of the knee, and some basic information, such as the weight of a patient. The modelling method is based on 20 years of research but is a trade secret.
“We are now in the process of commercialising the idea. Predicting the course of osteoarthritis is beneficial for patients who are up to 65 years old when it is still possible to affect the progression of the disease. Besides improving quality of life and bringing health benefits for individual patients, the innovation holds promise to significantly cut not only public health care costs but also occupational health care costs,” Turunen notes.
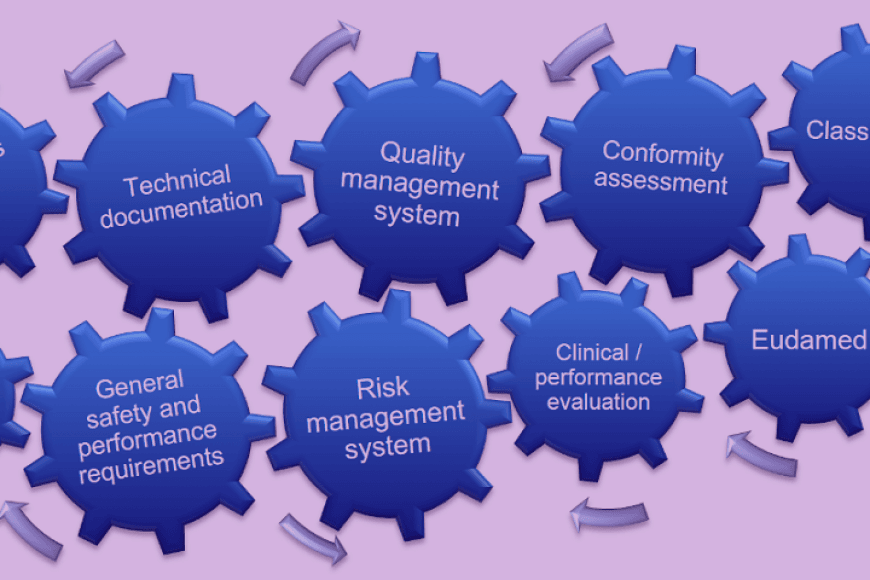
“Although we are not manufacturing an actual device, our software counts as a medical device. Everything that affects the treatment of patients falls within the scope of medical device regulations,” Turunen points out.
As accurate predictions enable the delivery of personalised treatments and motivate patients to follow their treatment plan, they are a useful tool for medical professionals. Algoa Progress is seeking to enter the European market and later expand to the USA. For most health tech companies, the market in Finland is too small. The software could be used in hospitals or even integrated into imaging devices. There are no similar products currently available in the market.
Documentation takes a great deal of work
New health tech companies quickly face robust regulatory scrutiny. The first step for Algoa Progress is to obtain CE marking, which requires extensive documentation that demonstrates conformity with the requirements. The regulatory training offered by Tampere University came in very handy, and Mikael Turunen enrolled on the programme in the autumn of 2020.
“Our team came to the conclusion that we need more regulatory expertise, and together we decided that I would be the one completing the training. We are planning to eventually hire a dedicated professional to manage this side of the business, but we are not there yet. Still, it was important to bring regulatory expertise on board at this point. We were surprised by the workload that comes with ensuring compliance and meeting the documentation requirements,” Turunen says.
The content of the training programme met his expectations, although delving into a new topic in a virtual environment took some getting used to.
“It has been a while since I took a course. I have taught physics and natural sciences, but this was a new experience for me as the training does not entail any calculations,” Turunen laughs.
The programme focused on building an overall understanding of multiple large-scale phenomena. Legal language may initially seem complicated but – owing to his academic background and prior experience in writing grant proposals – Turunen was already familiar with the type of language. He assures that it is possible to learn how to read and interpret standards and regulations.
“So many things can be easily made to sound more difficult and complicated than they actually are. If the regulatory compliance aspect of the business if outsourced, it can lead one to believe that the whole process is exceedingly difficult, but it can be mastered with some effort. And of course we can reduce costs at the R&D stage by doing this ourselves,” Turunen points out.
“The training programme has been well organised. The virtual sessions have been interactive, which has kept up interest. The first course may have even been a bit too basic for someone who is already familiar with these questions, but it served as an excellent introduction for me as I was perhaps the most inexperienced of all the participants,” he adds.
Turunen recommends the regulatory training offered by Tampere University to everyone who is or will be working in a regulated environment.
“Especially the course taught by Alpo Värri titled Standards, Interoperability and Regulation in Health Informatics made me realise what a complex and interrelated web the regulatory landscape really is. While some of the regulations may not be directly related to my own line of work, the training programme has shown me that they may still have a bearing on the business. I have also gained a broad understanding of the questions that we may still need to consider, and I now know where regulatory decisions are made. I still have to turn in the final essay before completing the programme. I have had to become familiar with a broad range of materials to be able to navigate the complex regulatory framework, but I want to do this right and there is still plenty of time,” Turunen says.
After completing the courses (Regulatory training on health and medical technology), Turunen feels he is well-prepared to deal with regulatory demands.
“Now I will know whether, for example, a certain type of technical documentation is required if we come across new regulatory issues. I have learned so many things I did not even know existed.”
Text: Annamari Karjalainen