Health technology solutions – product development and commercialization (programme)
Extent
15 ECTSCourse dates
Application period
Fees
Faculty or school
Language of instruction
Study fields
Organising unit
Mode of study
Study level
Extensive and up-to-date information package on regulations and requirements for product development and commercialization of health technology products
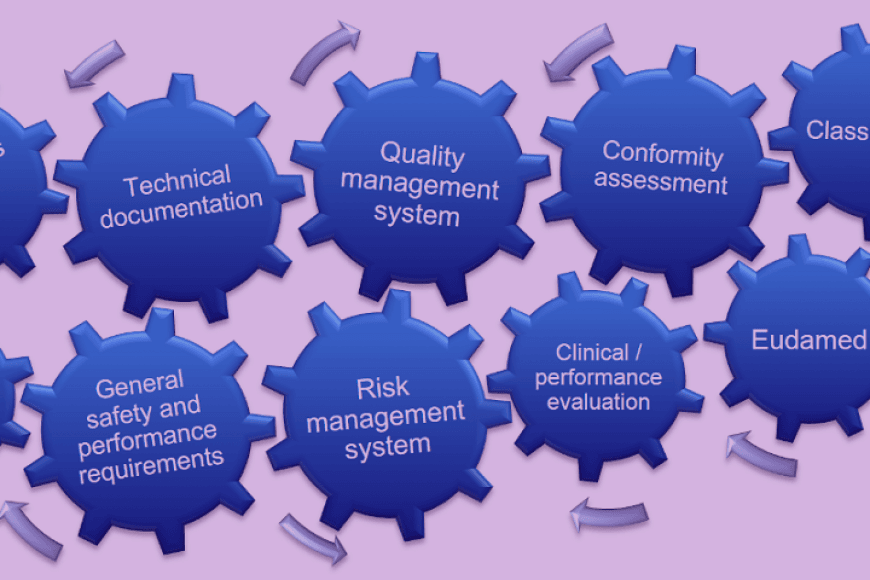
Health technology products are tightly regulated and controlled by the authorities. Up-to-date information on regulations, requirements and industry practices are the keys to successful product development and product commercialization. This programme offers you the opportunity to expand your knowledge and grow as an expert in health and medical technology.
The programme includes four courses. Two of them are compulsory and two are optional of which you may choose one. If completing the programme is not currently relevant to you, you can also take individual courses as needed.
Target group
This programme is targeted for those who are working or willing to work in health technology companies in different roles in R&D, quality and regulation, production, marketing and sales and management. The programme gives essential knowledge also for health technology start-ups and anyone who wants to expand his or her knowledge about requirements, regulations, and practices when developing and commercializing health technology products for global markets.
For those who would like to have a competence to be “Person responsible for regulatory compliance” this programme offers a formal additional education when you already have a university degree in law, medicine, pharmacy, engineering or another relevant scientific discipline or 4 years of professional experience in regulatory affairs or in quality management systems on medical devices.
Feedback and experiences from the programme
Regulatory training is necessary already at the R&D stage
PhD Mikael Turunen works at Algoa Progress which develops software for modelling the progression of osteoarthritis: “Although we are not manufacturing an actual device, our software counts as one. Everything that affects the treatment of patients falls within the scope of medical device regulations. It was important to bring regulatory expertise on board at this point."


Saara Hassinen, CEO of Healthtech Finland
“As for the implementation of the regulations, there are concerns over the availability of the required skills. After the regulations take effect, manufacturing companies will have to appoint someone to oversee compliance, and this person will also have significant responsibilities relating to quality management.”