Introduction to quality and regulation for health technology products
Extent
5 ECTSCourse dates
Application period
Fees
Faculty or school
Language of instruction
Study fields
Organising unit
Mode of study
Study level
Comprehensive online course on regulatory and quality requirements and practices for health technology products throughout the product lifecycle
When working with medical devices or other health technology products, it is important to know how this industry operates and what regulations and standards must be followed to get your product on the market.
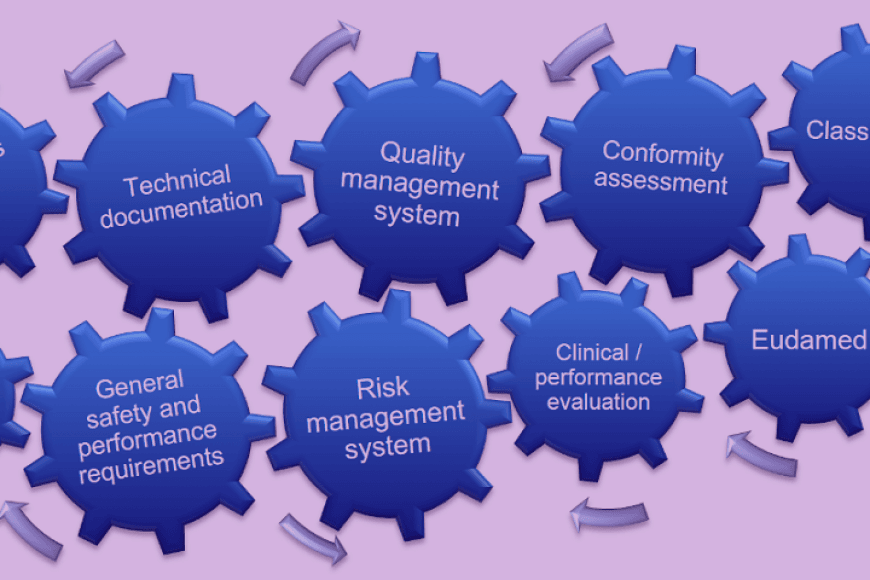
This online course gives you a comprehensive and up-to-date overview of global industry requirements, regulations, and practices. It introduces different types of health technology products, regulatory framework, stages of product lifecycle, and risk-based thinking. The key concepts and definitions in the field, versatile product testing, product safety verification, usability and related standards will be also introduced not forgetting the ethical issues and clinical evaluation.
Targer group
This course is targeted for those who are working or willing to work in health technology companies in different roles in R&D, quality and regulation, production, marketing and sales and management. The course gives essential knowledge also for health technology start-ups and anyone who wants to update his or her knowledge about requirements, regulations and practices related to health technology products.
The course can also be customized for a company or organization.
“This is an excellent opportunity for companies to support the development of expertise among their staff,” says Saara Hassinen, CEO of Healthtech Finland.
This course is part of Health technology solutions – product development and commercialization (programme). Get acquainted with the programme and other courses included in it.
The programme offers a formal additional education for a “Person responsible for regulatory compliance” required by the new EU regulations in Medical Devices Regulation (MDR) and In-vitro Diagnostics Regulation (IVDR).