Researchers investigate how the tuberculosis bacterium protects itself against antibiotics
“This is a significant finding for TB drug development,” says Associate Professor Mataleena Parikka from Tampere University, Finland, who led the study.
“TB drugs under development should not be tested on single-cell bacteria, but on bacteria that grow in a biofilm that protects the bacteria and are naturally tolerant to antibiotics,” says Parikka.
TB is an infectious disease caused by Mycobacterium tuberculosis and a major global health problem. Half a million people with M. tuberculosis are fully resistant to most common drugs.
Even with antibiotic-sensitive TB, treatment takes at least six months. Long antibiotic treatment reduces the symptoms of the disease, but the pathogen may still be viable and infectious at the end of treatment. Long and ineffective treatment promotes the development of genetically drug-resistant strains of TB bacteria.
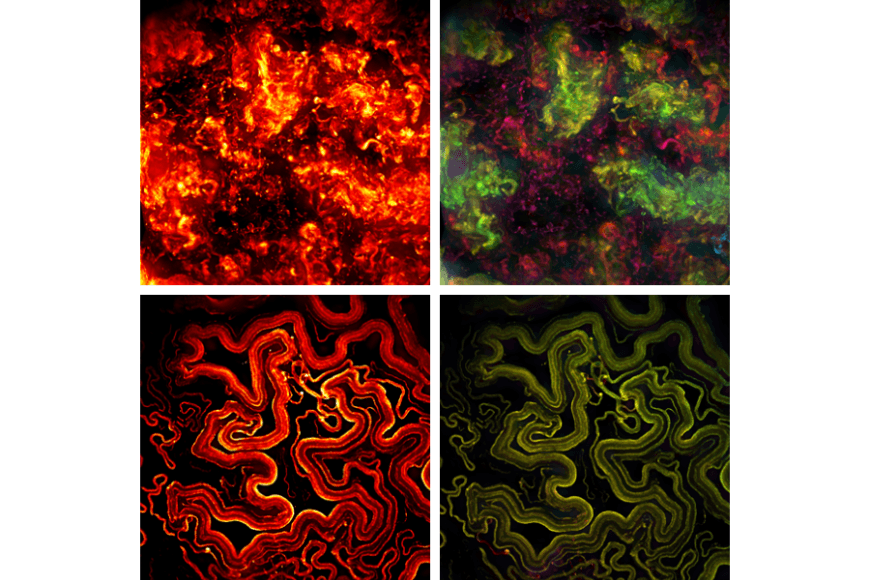
Only recently has it been shown that TB bacteria are protected from drugs and the human immune system by a biofilm that the bacterium forms when it grows as a community of several bacteria.
Cellulose produced by mycobacteria plays a key role
Work by researchers from the Universities of Tampere and Helsinki and Oslo University Hospital now showed that cellulose produced by mycobacteria in biofilm matrices is an important factor in maintaining biofilm structure and resistance to antibiotics. The bacterium secretes an enzyme that breaks down cellulose, and a reduction in this enzyme ‘triggers’ biofilm formation.
The study also found significant differences in virulence and antibiotic tolerance factors between bacteria growing in biofilms and planktonic bacteria.
One of the most important differences was related to the level of enzymes regulating cell division; mycobacterial cells divide asymmetrically and at different rates in the biofilm communities that were studied. This asymmetry results in bacterial cells that have different characteristics - including persister cells. Persister cells are dormant bacteria and bacterial cells with enhanced antibiotic tolerance due to changes in metabolism.
“We showed that even the young mycobacterial bacterial biofilm contains persister cells that are highly tolerant to antibiotics and survive the antibiotic treatment that kills normal cells,” says Parikka says.
Research teams are already developing drug therapies
“Our research teams are currently developing drug therapies targeting these protective mechanisms, with the aim of making mycobacteria sensitive to conventional TB antibiotics. These completely new types of therapies can shorten and improve the effectiveness of drug treatments for TB, and thus prevent the spread of genetic drug resistance,” says Parikka.
The study was funded by the Academy of Finland, Sigrid Jusélius Foundation, Jane and Aatos Erkko Foundation, and Tampere Tuberculosis Foundation.
Kirsi Savijoki, Henna Myllymäki, Hanna Luukinen, Lauri Paulamäki, Leena-Maija Vanha-Aho, Aleksandra Svorjova, Ilkka Miettinen, Adyary Fallarero, Teemu O Ihalainen, Jari Yli-Kauhaluoma, Tuula A Nyman, Mataleena Parikka. Surface-shaving proteomics of Mycobacterium marinum identifies biofilm subtype-specific changes affecting virulence, tolerance, and persistence. mSystems 2021 Jun 29;6(3):e0050021. http://doi.org/10.1128/mSystems.00500-21
Mataleena Parikka’s infection biology research group at Tampere University
Enquiries:
Associate Professor Mataleena Parikka, +358 (0)40 735 5052, mataleena.parikka [at] tuni.fi





